Full Text
Background
Cerebrovascular reactivity (CVR) measures the ability of the brain’s vasculature to respond to a vasodilatory stimulus, such as CO2 , and is an emerging imaging metric of cerebrovascular health. Breath-Hold (BH) induced CVR mapping is a valid alternative to gas inhalation challenges [1] with functional MRI. However, BH movement artifacts are time-locked with the vasodilatory signal of interest, potentially introducing considerable bias on CVR estimates. Multi-Echo (ME) BOLD fMRI enhances the sensitivity to the BOLD effect by optimally combining the echoes, and enables denoising approaches that have demonstrated to remove non-BOLD (e.g. movement) artefacts effectively [2-5]. Using a combined ME BOLD and pseudo-continuous arterial spin labelling acquisition, Cohen and Wang have shown that Optimal Combination (OC) of ME time series improves the reliability and repeatability of BH induced CVR mapping [6] assessed over 2 sessions. However, OC is not sufficient to remove motion effects from true fluctuations related to CVR. Here, we (1) generalise these results by computing quantitative CVR maps obtained from a fast (1.5 s) TR, ME-BOLD acquisition acquired over 9 sessions with concurrent end-tidal CO2 recordings, and (2) compare how single echo, OC and ME-based Independent Component Analysis (ME-ICA) denoising [3,4,5] preprocessing pipelines remove motion artefacts and obtain more reliable results.
Methods
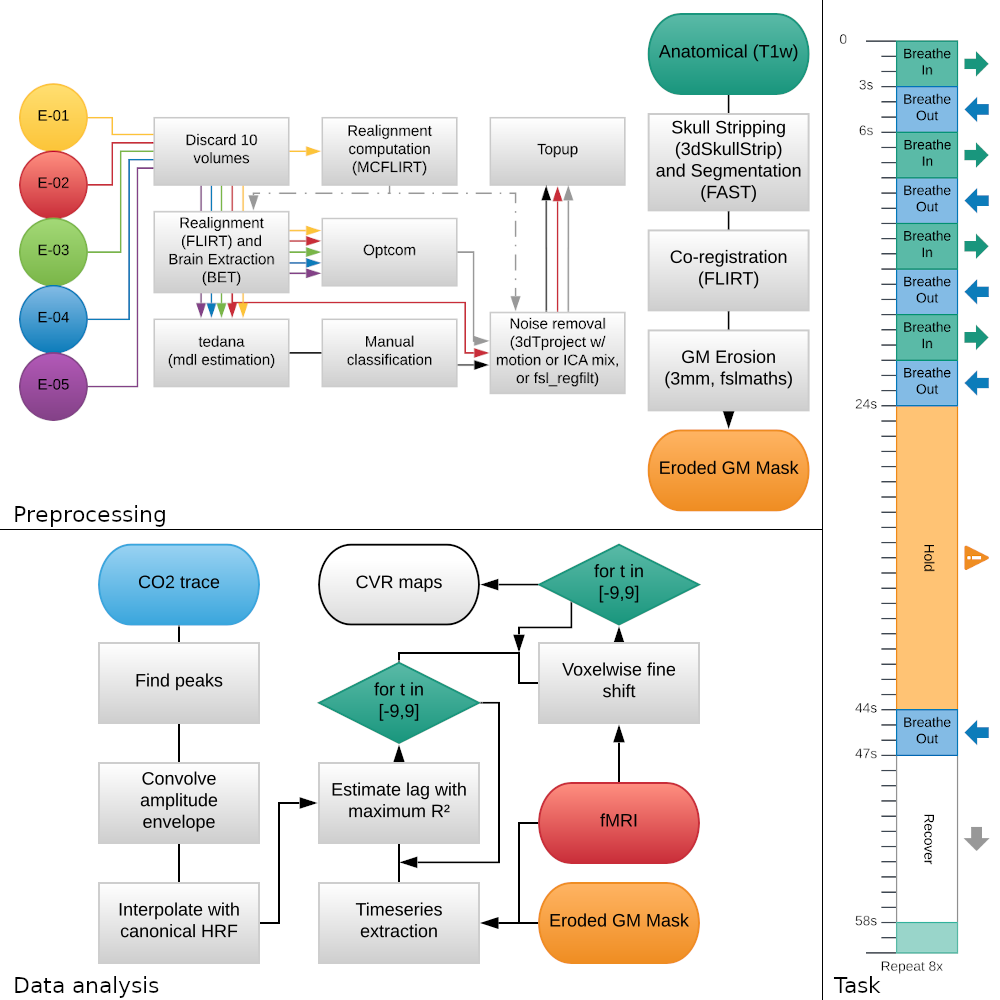
Seven healthy volunteers underwent 10 MRI sessions in a 3T Siemens PrismaFit scanner, spaced 1-week apart at the same time of day. A BH task (Fig. 1) adapted from [7] was administered at each session while collecting ME-fMRI data (340 scans, TR=1.5 s, TEs=10.6/28.69/46.78/64.87/82.96 ms, flip angle=70°, MB=4, GRAPPA=2, 52 slices, Partial-Fourier=6/8, FoV=211x211 mm2, voxel size=2.4x2.4x3 mm3). CO2 levels were measured using a nasal cannula, a gas analyzer (ADInstruments), and a BIOPAC MP150 system. Data preprocessing and data analysis followed the same steps reported in [8]. Briefly, after a minimal data preprocessing (Fig. 2), CVR and lag maps were first obtained in the second TE (SE) and OC data (GLM with lagged HRF-convolved PETCO2 timeseries as covariate of interest; 12 motion parameters and Legendre polynomials as nuisance regressors, SE-MPR or OC-MPR, Fig. 3). The optimally combined volume was decomposed using ME-ICA [9], then noise and signal ICs were manually labelled, and three additional extended GLMs were created: adding the noise ICs timeseries directly as additional nuisance regressors (ME-AGG); orthogonalized w.r.t. the PETCO2 trace (ME-MOD); or orthogonalised w.r.t. the PETCO2 trace and signal ICs timeseries (ME-CON). FD [10] and DVARS [11] were computed before realignment (SE-PRE) and after removal of the fitted nuisance regressors from SE and OC data. The CVR and lag maps were registered to the MNI space, then ICC(2,1) [12] was computed to assess their reliability across sessions.
 Figure 1: Left: Preprocessing and data analysis used in this study. Right: BreathHold task.
Figure 1: Left: Preprocessing and data analysis used in this study. Right: BreathHold task.
Results
Considering the relationship between FD and DVARS (Fig. 4-5) and the ICC analysis (Fig. 6), a conservative ME-ICA denoising approach is the best way to reduce impact of motion without compromising reliability in CVR mapping. Otherwise, a conventional OC approach is recommended, but with less reduction of motion effects. Further studies should extend these results to other fMRI with substantial collinear artefacts.
 Figure 2: CVR map for each denoising and session of a representative subject
Figure 2: CVR map for each denoising and session of a representative subject
 Figure 3: Lag map for each denoising and session of a representative subject
Figure 3: Lag map for each denoising and session of a representative subject
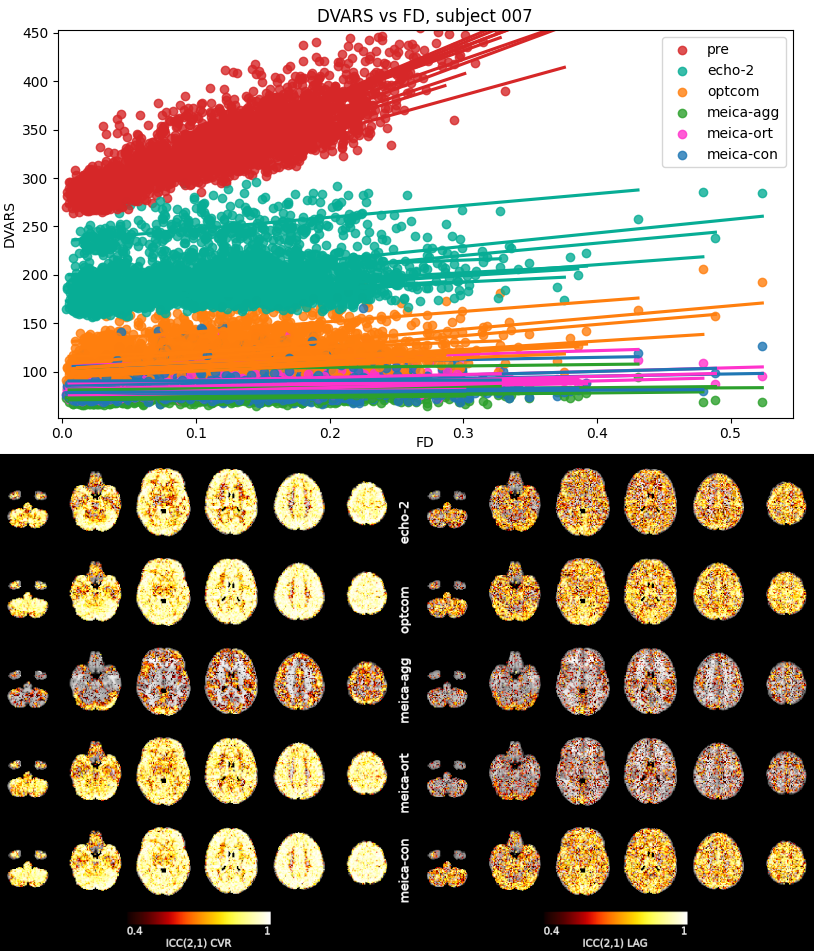
 Figure 4: FD vs DVARS correlation for a representative subject, and (B) for all subjects for all of the analysis approaches. A lower
slope in the correlation means lower residual motion effects and better motion denoising.
Figure 4: FD vs DVARS correlation for a representative subject, and (B) for all subjects for all of the analysis approaches. A lower
slope in the correlation means lower residual motion effects and better motion denoising.
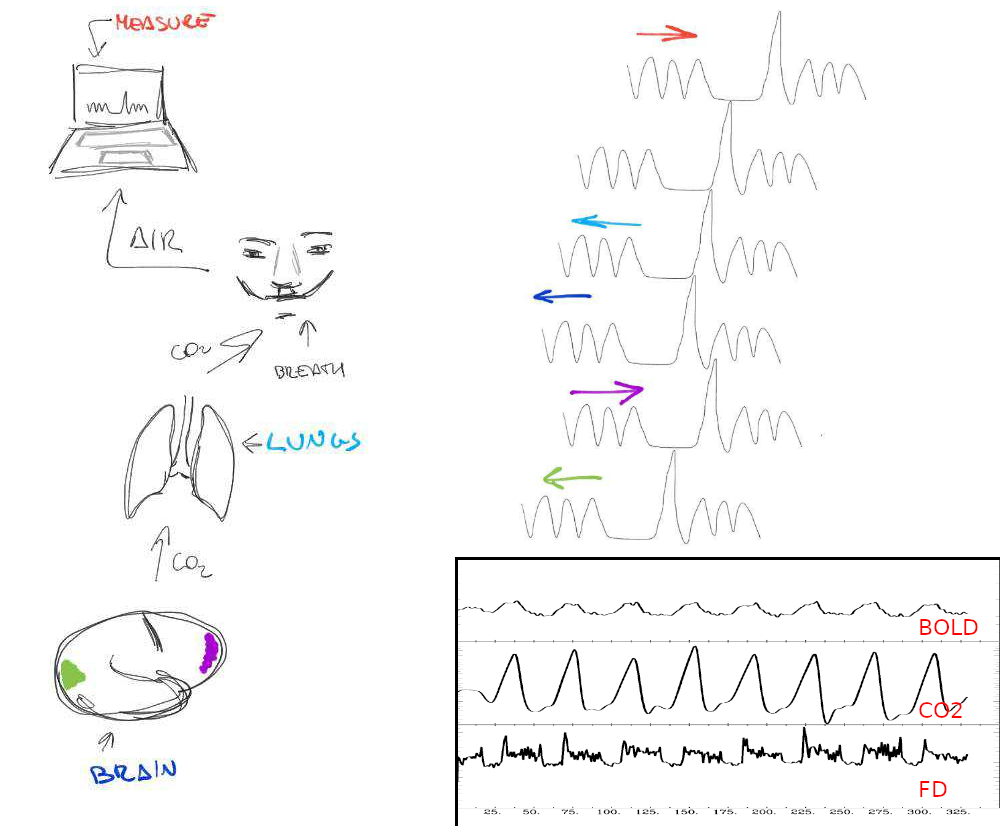
 Figure 5: Right: BH responses in %DVARS and %BOLD. Left: Tendency to average in all the trials.
Figure 5: Right: BH responses in %DVARS and %BOLD. Left: Tendency to average in all the trials.
 Figure 6: Thresholded (>0.4) voxelwise ICC(2,1) of CVR and lag in MNI space. Note how optcom and meica-con have the best reliability
for both CVR and lag maps, while meica-agg has the lowest in both. ICC below 0.4 indicates poor reliability.
Figure 6: Thresholded (>0.4) voxelwise ICC(2,1) of CVR and lag in MNI space. Note how optcom and meica-con have the best reliability
for both CVR and lag maps, while meica-agg has the lowest in both. ICC below 0.4 indicates poor reliability.
References
- Kastrup, A. (2001), ‘Assessment of cerebrovascular reactivity with functional magnetic resonance imaging: Comparison of CO2 and breath holding’, Magnetic Resonance Imaging, vol. 19, n. 1, pp. 13-20
- Caballero-Gaudes, C. (2017), ‘Methods for cleaning the BOLD fMRI signal’, Neuroimage, vol. 154, pp. 128-149
- Kundu, P. (2012) ‘Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI’, Neuroimage, vol. 60, pp. 1759–1770
- Kundu, P. (2013) ‘Integrated strategy for improving functional connectivity mapping using multiecho fMRI’, Proceedings of the National Academy of Sciences, vol. 110, n. 40, pp. 16187-16192
- Power, J.D. (2017), ‘Ridding fMRI data of motion-related influences: Removal of signals with distinct spatial and physical bases in multiecho data‘, PNAS, vol. 115, n. 9, E2105-14
- Bright, M.G. (2013), ‘Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance’, Neuroimage, vol. 83, pp. 559-568
- DuPre, E. (2019) ‘ME-ICA/tedana: 0.0.6’, doi:10.5281/ZENODO.2558498
- Moia, S., Stickland, R.C. (2020), ‘Voxelwise optimization of hemodynamic lags to improve regional CVR estimates in breath-hold fMRI’, accepted in Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Montreal, Canada;
- Power, J. D. (2012) ‘Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion’, Neuroimage, vol. 59, n. 3, pp. 2142–2154
- Smyser, C. D. (2011) ‘Functional connectivity MRI in infants: Exploration of the functional organization of the developing brain’, Neuroimage, vol. 56, n. 3, pp. 1437-1452
- Chen, G. (2018) ‘Intraclass correlation: Improved modeling approaches and applications for neuroimaging’, Human Brain Mapping